Abstract
Background: Pevonedistat (PEV) is an inhibitor of NEDD8-activating enzyme (NAE) which is critical for degradation of several cellular proteins essential for tumor growth and survival. The combination of azacitidine (AZA) and venetoclax (VEN) is approved for patients (pts) with newly diagnosed (ND) AML who are unsuitable for intensive chemotherapy, and clinical studies show preliminary efficacy with AZA+VEN in higher risk MDS pts. We evaluate the efficacy and safety of the combination of AZA, VEN, and PEV in ND secondary AML (s-AML) and in MDS after HMA failure.
Methods: This phase 1/2 trial enrolled pts with ND s-AML, including therapy-related AML (t-AML), AML from prior MDS or chronic myelomonocytic leukemia (CMML) or myeloproliferative neoplasm (MPN), or AML with dysplasia in ≥50% cells in ≥2 myeloid lineages (MRC). A second arm enrolled pts with MDS or CMML that had progressed on prior HMA therapy. Pts had to have a performance status ≤2, total bilirubin ≤1.5 x upper limit of normal (ULN), ALT/AST ≤2.5 x ULN, and creatinine clearance ≥30 mL/min. Pts in AML arm may have received prior HMA for preceding hematologic malignancy. In both AML and MDS/CMML cohorts, pts received AZA 75 mg/m2 SC/IV on days 1-7 and PEV 20 mg/m2 IV on days 1, 3 and 5 on every 28 day-cycle. In AML cohort, VEN was given on days 1-28 in cycle 1 and days 1-21 on cycle 2+. In MDS/CMML cohort, VEN was given on days 3-14 in cycle 1 and days 1-14 on cycle 2+. In initial phase 1 portion of the study (AML cohort only), VEN at max dose 200 mg and 400 mg were explored.
Results: Between 3/2019 and 8/2021, 32 pts with AML (10 t-AML, 22 AML from MDS/CMML/MPN, 4 MRC) and 8 pts with MDS/CMML post-HMA failure (6 with MDS, 2 with CMML) were treated (Table). In the AML cohort, the median age was 74 years, with 14 pts (44%) ≥75 years of age. Cytogenetics was adverse in 21 pts (66%), and adverse risk mutations, including TP53, RUNX1, and ASXL1 were present in 34%, 31%, and 22%, respectively. 17 pts (53%) had treated secondary AML (ts-AML; i.e. prior exposure to HMA or chemotherapy for preceding myeloid neoplasm).
The overall response rate (ORR, defined as CR+CRi+MLFS) was 75%, with CR in 15 pts (47%), CRi in 6 pts (19%), and MLFS in 3 pts (9%). One pt achieved PR (3%). 78% of pts achieved best response after 1 cycle of therapy (range, 1-4 cycles to best response). Among 21 pts with CR/CRi, 10 pts (48%) achieved MRD negativity by flow cytometry. 14/21 pts (67%) with poor-risk cytogenetics responded, compared to 10/11 pts (91%) with non-poor cytogenetics. 9/11 pts (82%) with TP53-mutated AML responded. 11/17 pts (65%) with ts-AML responded, compared with 13/15 pts (87%) who did not have ts-AML.
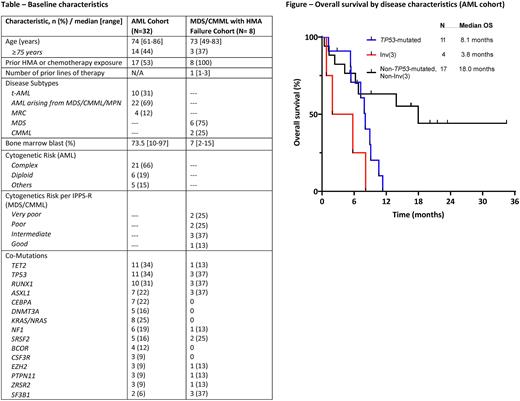
With median follow up of 20 months (mos) for the AML cohort, the median OS was 8.2 mos and median relapse-free survival (RFS) was 7.4 mos. 5 pts proceeded to transplant in first remission. The median OS for pts with adverse cytogenetics was 6.8 mos and was not reached in pts without adverse cytogenetics (1-year OS of 70%). The median OS for pts with TP53-mutated AML (n=11) was 8.1 mos, pts with inv(3) (n=4) was 3.8 mos, and pts without TP53 mutation or inv(3) (n=17) was 18 mos (Figure).
In MDS/CMML HMA failure cohort, the median age was 73 years. Most pts had adverse cytogenetics (50%) and were high to very high-risk by IPSS-R (87%). Overall response rate (CR+marrow CR [mCR]+HI) was 75%, with CR in 3 pts (37%), mCR in 2 pts (25%), and HI in 1 pts (13%). With a median follow up of 11 mos, the median OS was 9.9 mos. Transformation to AML occurred in 1 pt (after 11 mos).
No DLTs were observed in the phase 1 portion, and VEN 400 mg daily was the recommended phase 2 dose. Myelosuppression was similar to expectations with AZA+VEN alone. Grade 3/4 non-hematologic adverse events occurring in ≥10% pts were febrile neutropenia or other infection in 75%, hypophosphatemia in 22%, and hyperbilirubinemia in 10%. The 4-week and 8-week mortality rates were 9% and 25% in AML cohort and 0% and 13% in MDS/CMML cohort, respectively.
Conclusion: AZA, VEN, and PEV combination is active and well-tolerated in both ND s-AML and MDS/CMML after HMA failure. This population was enriched with very poor risk features, including a majority of pts in the AML cohort with prior HMA or chemotherapy exposure for an antecedent myeloid malignancy and 1/3 of pts with a TP53 mutation. Response rates were high across disease subgroups, although duration of responses and survival were modest in pts with historically poor risk features, including those with adverse risk cytogenetics and TP53 mutations.
Disclosures
Garcia-Manero:Aprea: Honoraria; Genentech: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Gilead Sciences: Research Funding; AbbVie: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Curis: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding. Alvarado:BerGenBio: Research Funding; FibroGen: Research Funding; Sun Pharma: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; Jazz Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding. Konopleva:Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy; Forty-Seven; F. Hoffman LaRoche: Honoraria; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding. Jabbour:Pfizer: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding. Jain:Pfizer: Research Funding; MEI Pharma: Honoraria; TG Therapeutics: Honoraria; Servier Pharmaceuticals LLC: Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; TransThera Sciences: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; CareDx: Honoraria; Ipsen: Honoraria; Medisix: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Beigene: Honoraria; Loxo Oncology: Research Funding; Incyte Corporation: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; ADC Therapeutics: Research Funding; Takeda: Research Funding; Cellectis: Honoraria, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Novalgen: Research Funding; Newave: Research Funding; Mingsight: Research Funding; Dialectic Therapeutics: Research Funding; Cellectis: Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding. Borthakur:Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. DiNardo:Cleave: Research Funding; Forma: Research Funding; Astex: Research Funding; Takeda: Honoraria; LOXO: Research Funding; Servier: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; AbbVie: Consultancy, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Jazz: Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; Bluebird Bio: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Foghorn: Honoraria, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Issa:Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding; Novartis, Kura Oncology, Nuprobe: Consultancy. Sasaki:Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka Pharmaceuticals: Honoraria. Takahashi:Symbio Pharmaceuticals: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy; GSK: Consultancy; Agios: Consultancy; Mission Bio: Honoraria; Ostuka Pharmaceuticals: Honoraria; Illumina: Honoraria. Andreeff:CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Chimerix: Current holder of stock options in a privately-held company; Reata: Current holder of stock options in a privately-held company; NCI: Membership on an entity's Board of Directors or advisory committees; Oxford Biomedical UK: Research Funding; German Research Council: Membership on an entity's Board of Directors or advisory committees; Kintor Pharmaceutical: Research Funding; Pinot Bio: Research Funding; Brooklyn ITX: Research Funding; Syndax: Consultancy, Research Funding; Senti Bio: Consultancy, Research Funding; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Consultancy; Medicxi: Consultancy; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Breast Cancer Research Foundation: Research Funding; Oncolyze: Current holder of stock options in a privately-held company; Daiichi-Sankyo Inc.: Consultancy, Research Funding. Kantarjian:Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Cortes:Bristol Myers Squibb: Consultancy, Research Funding; Gilead: Consultancy; Forma Therapuetic: Consultancy; Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Sun Pharma: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Kartos: Research Funding. Short:AstraZeneca: Consultancy; Stemline Therapeutics: Research Funding; Amgen: Consultancy, Honoraria; Novartis: Consultancy; Astellas: Research Funding; Pfizer: Consultancy; Takeda Oncology: Consultancy, Research Funding.
OffLabel Disclosure:
Venetoclax is not approved for MDS or CMML. Pevonedistat is not approved for AML, MDS or CMML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal